
A deeper look into SMA
Treated at ~2½ months old
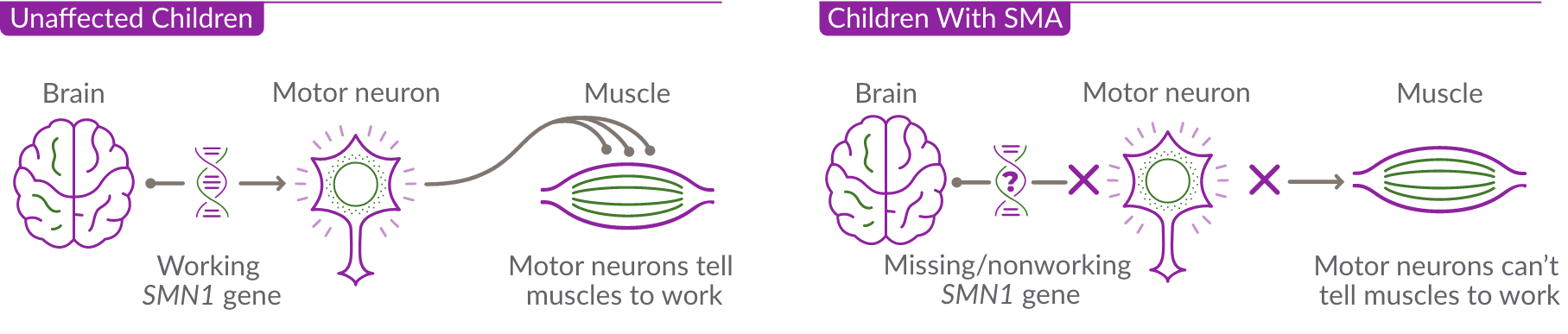
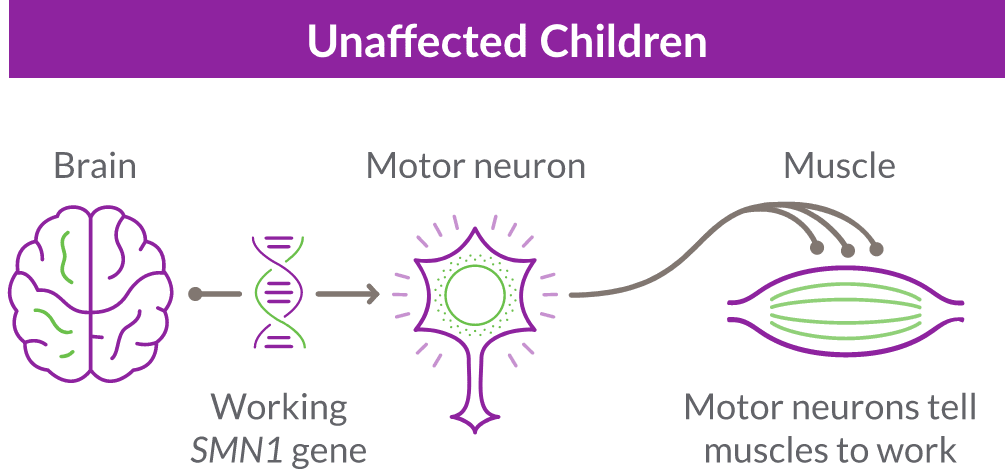
SMA, or spinal muscular atrophy, is a progressive, rare genetic disease that affects the motor nerve cells in the spinal cord and impacts the muscles used for breathing, eating, crawling, and walking.
What causes SMA?
Missing or nonworking SMN1 gene
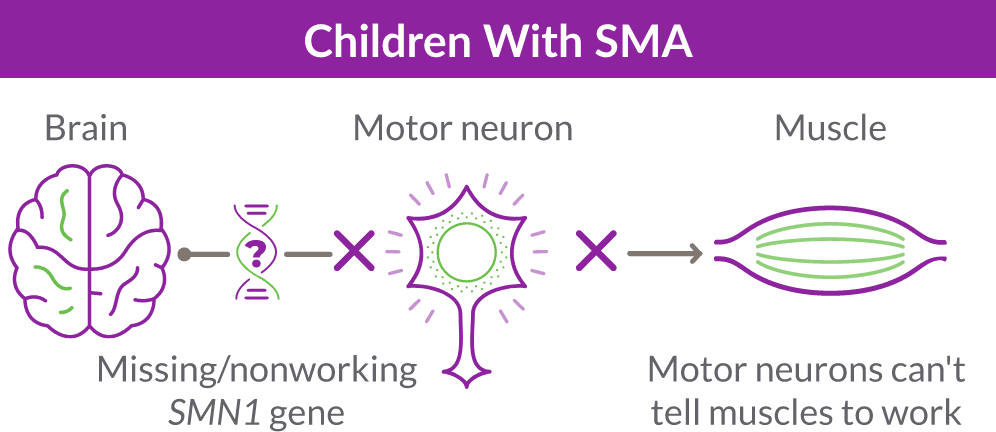
SMA is caused by the survival motor neuron 1 (SMN1) gene, which is missing or not working properly. This gene produces the survival motor neuron (SMN) protein that is critical to the nerves that control our muscles.
When the SMN1 gene is missing or not working properly, the body cannot make enough SMN protein. Without enough SMN protein, select motor neuron cells throughout the body may lose their ability to function and die. As a result, people with SMA experience muscle weakness and may develop difficulty moving, breathing, swallowing, or speaking.
The role of the SMN2 backup gene
Like many genes, the SMN1 gene has a backup gene, called the survival motor neuron 2, or SMN2 gene. In people with SMA, when the SMN1 gene is missing or not working properly, the SMN2 gene can help produce SMN protein.
For people with SMA, the SMN2 gene is the main source of SMN protein production; however, it makes only about 10% of working SMN protein. ZOLGENSMA® (onasemnogene abeparvovec-xioi) delivers a new, working SMN gene to the body's cells, which then replaces the SMN1 gene. This allows the cells—particularly motor neuron cells—to produce SMN protein.
People can have 1 or more copies of this backup gene and usually, the more copies of the SMN2 gene a person has, the less severe his or her SMA is. However, even people with several copies of the SMN2 gene may not produce as much SMN protein as they need, so their motor neuron cells may not work as they should.
Read more about how gene therapy treatment can stop SMA progression
Learn about SMA treatment with ZOLGENSMA
Detecting and diagnosing SMA
Staying ahead of progression
Today, SMA is usually discovered during a newborn screening. If a child tests positive for SMA, the doctor will order genetic tests to confirm the diagnosis and to see how many copies of the SMN2 backup gene the child has. They may perform additional tests to detect genetic mutations, which could indicate that the SMN1 gene is not working properly.
Whether the SMN1 gene is missing or not working, the more copies of SMN2 a person has, the less severe SMA is. And doctors can identify the number of copies before a baby shows any symptoms, which is important for early treatment.
For example, if left untreated, a baby with 2 copies of SMN2 may show symptoms sooner than a baby with 4 copies.

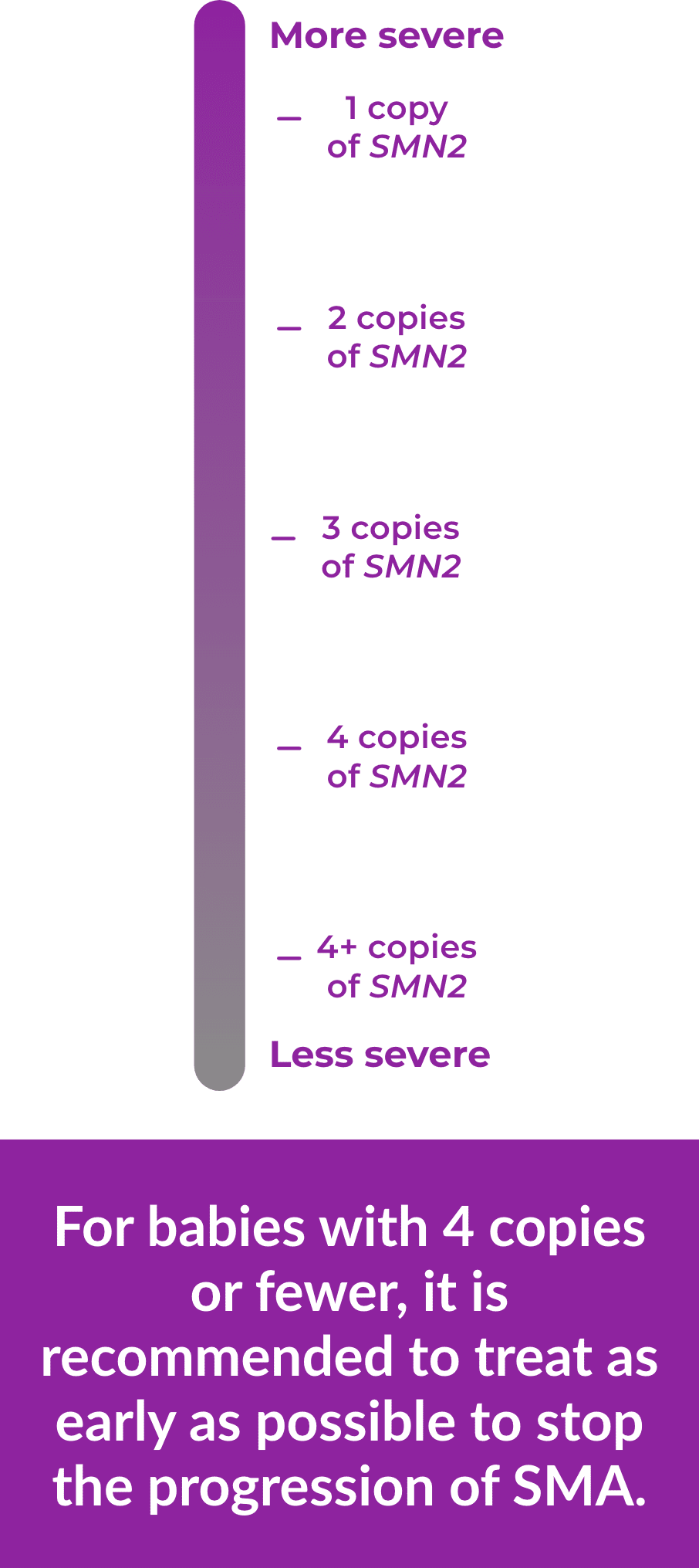
For babies with 4 copies or fewer, it is recommended to treat as early as
possible to stop the progression of SMA.
Before newborn screening, most children weren’t diagnosed until they showed symptoms. They were given a “type” of SMA, which classifies the severity of the disease. A child’s type was determined based on when symptoms first appeared and which motor milestones they had reached. There are four main types of SMA (Types 1-4). SMA Type 1 is the most common and is very serious. There is an additional fifth type called SMA Type 0. This is the most severe type and can be fatal before the child is born. Now that newborn screening is routine, most children are diagnosed before they are assigned a type. If you believe your child is showing symptoms of SMA but has not been diagnosed, talk to your doctor immediately or visit CureSMA.org
Due to the latest technological advances in science, doctors are able to detect SMA early and can treat before SMA can progress. For more information about types of SMA, visit CureSMA.org
How children are diagnosed
Newborn screening
Early diagnosis before signs appear through newborn screening, and early intervention with available treatments lead to better outcomes. This is especially true with SMA, where early detection and timely administration of therapies can prevent the rapid and irreversible loss of motor function caused by the disease. Today, all 50 states and Washington, DC screen newborns for SMA at birth; 100% of babies born in the United States are screened in this manner.
Prenatal testing
Prenatal screening can determine if a fetus has inherited SMA and can help families prepare for treatment as early as possible. Prenatal tests for SMA include amniocentesis and chorionic villus sampling (CVS).
Showing signs of SMA
In some states, newborn screening is not routine and SMA is diagnosed when a child begins to show signs of the disease.
How SMA is inherited
SMA is an autosomal recessive disorder. This means that in order to have SMA, a person must have 2 copies of a nonworking SMN1 gene or be missing both copies of the SMN1 gene.
What is a carrier?
A carrier is a person who has 1 healthy copy and 1 nonworking copy of the SMN1 gene. A carrier generally does not show any signs or symptoms of SMA but they may pass the nonworking gene on to their children.
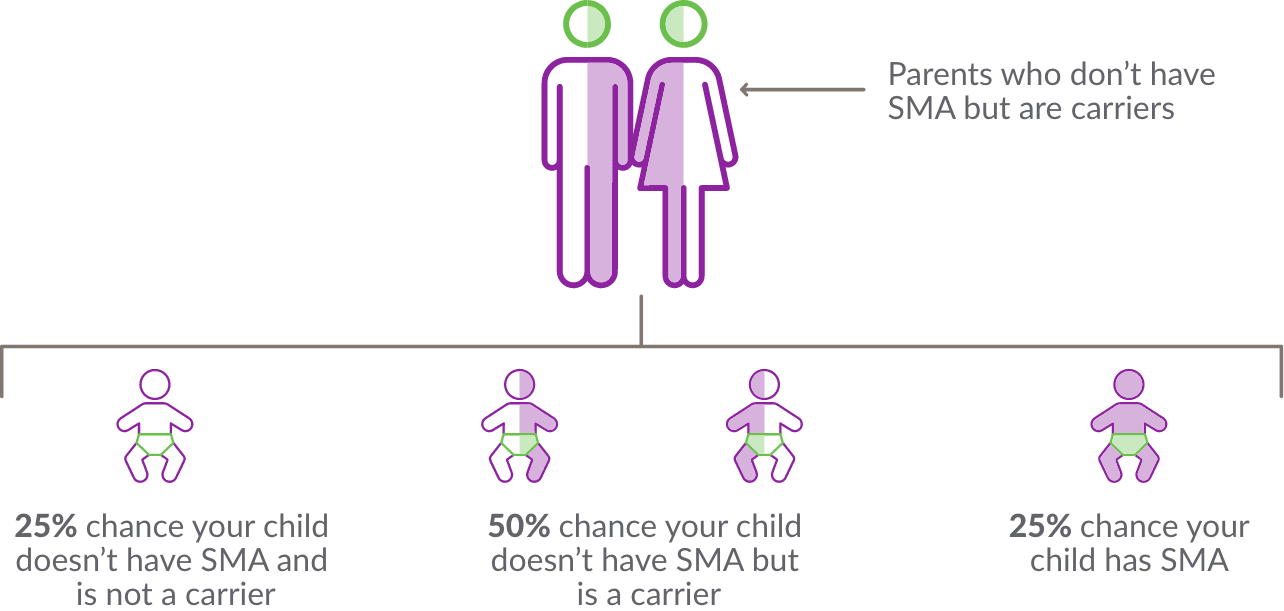
How can someone find out if they are a carrier?
A genetic test is the only way to know if a person is a carrier of SMA. This is done through a blood test. Partners who are thinking about becoming pregnant, or who are already pregnant can get a carrier screening for SMA and other genetic conditions. People with a family history of SMA should consider being tested. If you are interested in learning more about carrier testing, speak to a genetic counselor or your doctor.